- Our Work /
- Research Clinical Trials
Research Clinical Trials
This was a solution that rebuilt an existing web app using modern architecture and design. The goal was to provide a secure environment for researchers and a user-friendly experience for participants. Research Clinical Trials allows everyone to contribute to advancements in healthcare. We want to be a part of that.
We built a completed and working prototype solution that had a front-end web app, secure enclave SQL database, and a HL7 FHIR rapid exchange for healthcare data. This was a proof-of-concept pilot used to generate requirements' docs and security standards that could be shared. This pilot was a success, and we have since archived the resources.
Clinical trials are research studies that test a medical, surgical, or behavioral intervention in people. These trials are the primary way that researchers determine if a new form of treatment or prevention, such as a new drug, diet, or medical device (for example, a pacemaker), is safe and effective in people.
The previous solutions looked at the web app and services from the researchers' point of view and did not include user-flow for patients to easily navigate these trials. This project was about focusing both on researchers and trial participants.
Proof-of-Concept Development
Creating secure, compliant, and user-friendly healthcare applications requires careful planning and validation through proof-of-concept builds.
The Value of Proof-of-Concept Builds
For complex healthcare applications, proof-of-concept builds allow teams to validate technical approaches before committing to full-scale development. This approach offers several advantages:
- Early identification of technical challenges and security considerations
- Validation of compliance with healthcare regulations like HIPAA
- Demonstration of key functionality to stakeholders for feedback
- Creation of detailed requirements and security standards documentation
- Risk mitigation before significant development investment
Our Proof-of-Concept Approach
For the Research Clinical Trials platform, we developed a functional prototype with all critical components:
Frontend Application
A Progressive Web App (PWA) built with Angular, providing responsive interfaces for both researchers and participants with role-based access control.
Secure Database Architecture
Implemented Azure SQL with Secure Enclave for protected health information (PHI) storage with encryption at rest and in transit.
HL7 FHIR Integration
Connected to healthcare systems using the HL7 FHIR standard for secure, standardized data exchange with existing EHR systems.
Authentication & Authorization
Integrated Auth0/Okta with Azure Active Directory for secure identity management and role-based access control.
By validating these components in a working prototype, we were able to demonstrate feasibility, document security standards, and establish a strong foundation for future development.
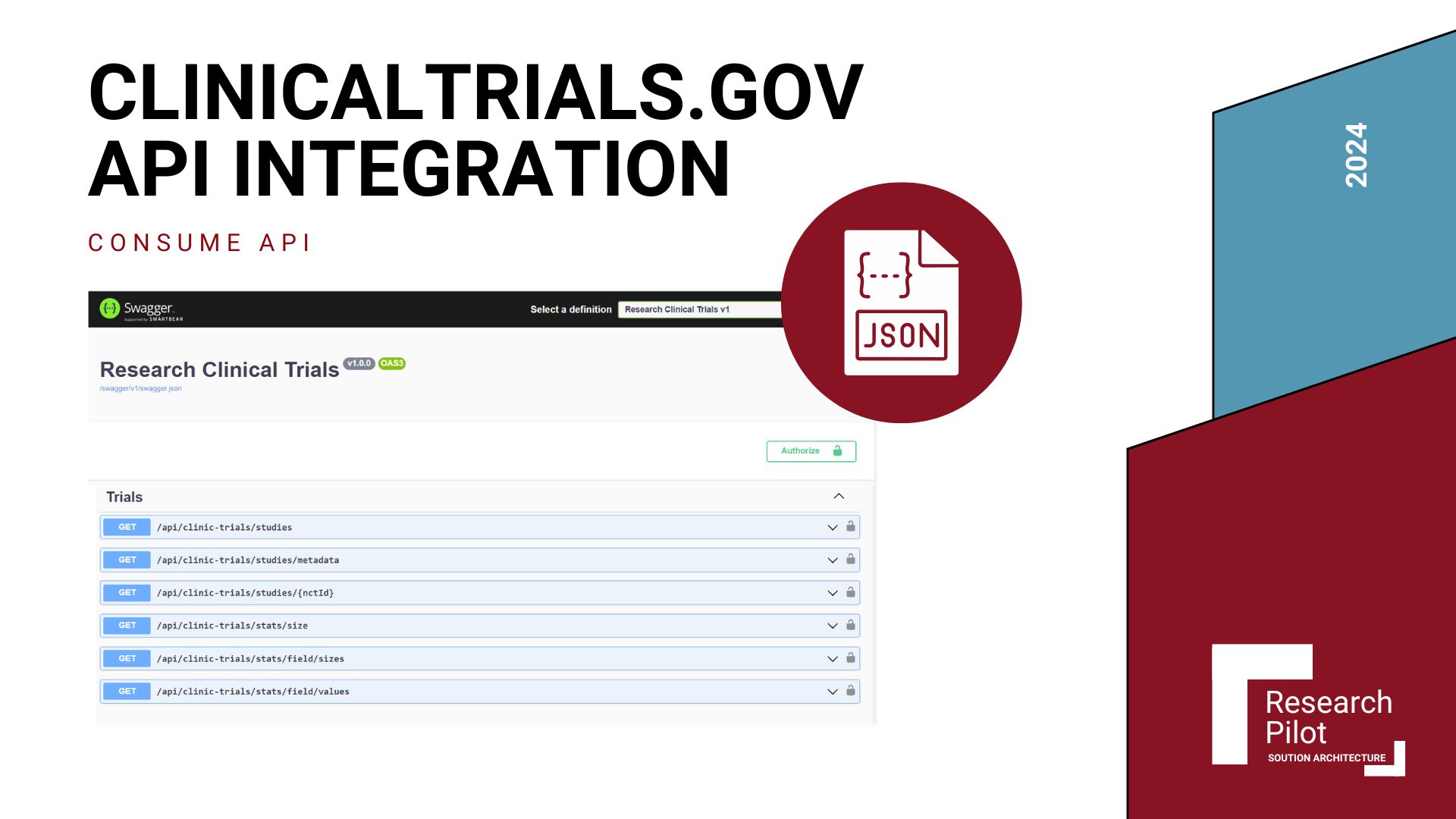
HL7 FHIR Services Integration
Fast Healthcare Interoperability Resources (FHIR) is a standard for healthcare data exchange that enables seamless integration across different healthcare systems.
What is HL7 FHIR?
HL7 FHIR (Fast Healthcare Interoperability Resources) is a next-generation standards framework created by Health Level Seven International (HL7) that defines how healthcare information can be exchanged between different systems.
FHIR uses modern web technologies like RESTful APIs, JSON, and XML to facilitate the exchange of healthcare data, making it easier to build applications that can connect to existing healthcare systems.
FHIR Integration in Our Research Platform
We integrated Azure FHIR Service into our clinical trials platform to enable secure, standardized healthcare data exchange, providing several critical benefits:
Interoperability
Connects clinical trial data with existing hospital Electronic Health Record (EHR) systems, allowing for seamless data exchange and reducing duplicate data entry.
Standardized Data
Uses common data models for patient information, medications, observations, and procedures that conform to healthcare industry standards.
Secure Data Exchange
Implements OAuth 2.0 authentication and SMART on FHIR protocols to ensure that patient data is securely transmitted with proper authorization.
Compliance
Ensures adherence to healthcare data standards and regulations, including HIPAA requirements for data privacy and security.
Technical Implementation
// Example FHIR Patient Resource
{
"resourceType": "Patient",
"id": "example",
"identifier": [
{
"system":
"https://research-trials.org/participants",
"value": "12345"
}
],
"active": true,
"name": [
{
"use": "official",
"family":
"[Redacted]",
"given":
["[Redacted]"]
}
]
}
We used Azure API for FHIR to handle FHIR resources, combined with Azure Functions to process and transform data between our internal data model and the FHIR standard.
Designing for Different Perspectives
Traditional clinical trial platforms focus primarily on researchers' needs. Our approach considered both researchers and patients to create a more balanced ecosystem.
Researcher Perspective
Needs & Priorities
- Efficient participant recruitment to meet study timelines
- Data management tools for tracking study outcomes
- Streamlined regulatory compliance and reporting
- Secure management of sensitive health data
Traditional Platform Limitations
- Complex interfaces focused on data collection
- Limited patient engagement capabilities
- Siloed data with minimal integration
Our Solution
We enhanced researcher capabilities with intuitive trial management dashboards, automated participant matching algorithms, and integrated compliance tools, all while maintaining secure access to patient data through proper authorization controls.
Patient Perspective
Needs & Priorities
- Finding relevant trials for specific conditions
- Understanding eligibility requirements
- Simplified participation process
- Privacy and data security assurances
Traditional Platform Limitations
- Difficult to discover relevant trials
- Complex medical terminology and jargon
- Limited transparency about trial process
Our Solution
We designed patient-centered features including condition-based trial matching, simplified eligibility screening, plain-language trial descriptions, and transparent consent processes, empowering patients to find and participate in relevant clinical research.
Benefits of a Balanced Approach
Improved Recruitment
By designing for patients' needs, the platform significantly increases trial visibility and participant enrollment rates, helping studies reach full enrollment faster.
Better Engagement
Clear communication and user-centered design reduces participant confusion and dropout rates, leading to more complete data collection and study adherence.
Accelerated Research
By serving both researcher and patient needs, the platform ultimately helps accelerate the clinical research process, potentially bringing new treatments to market faster.
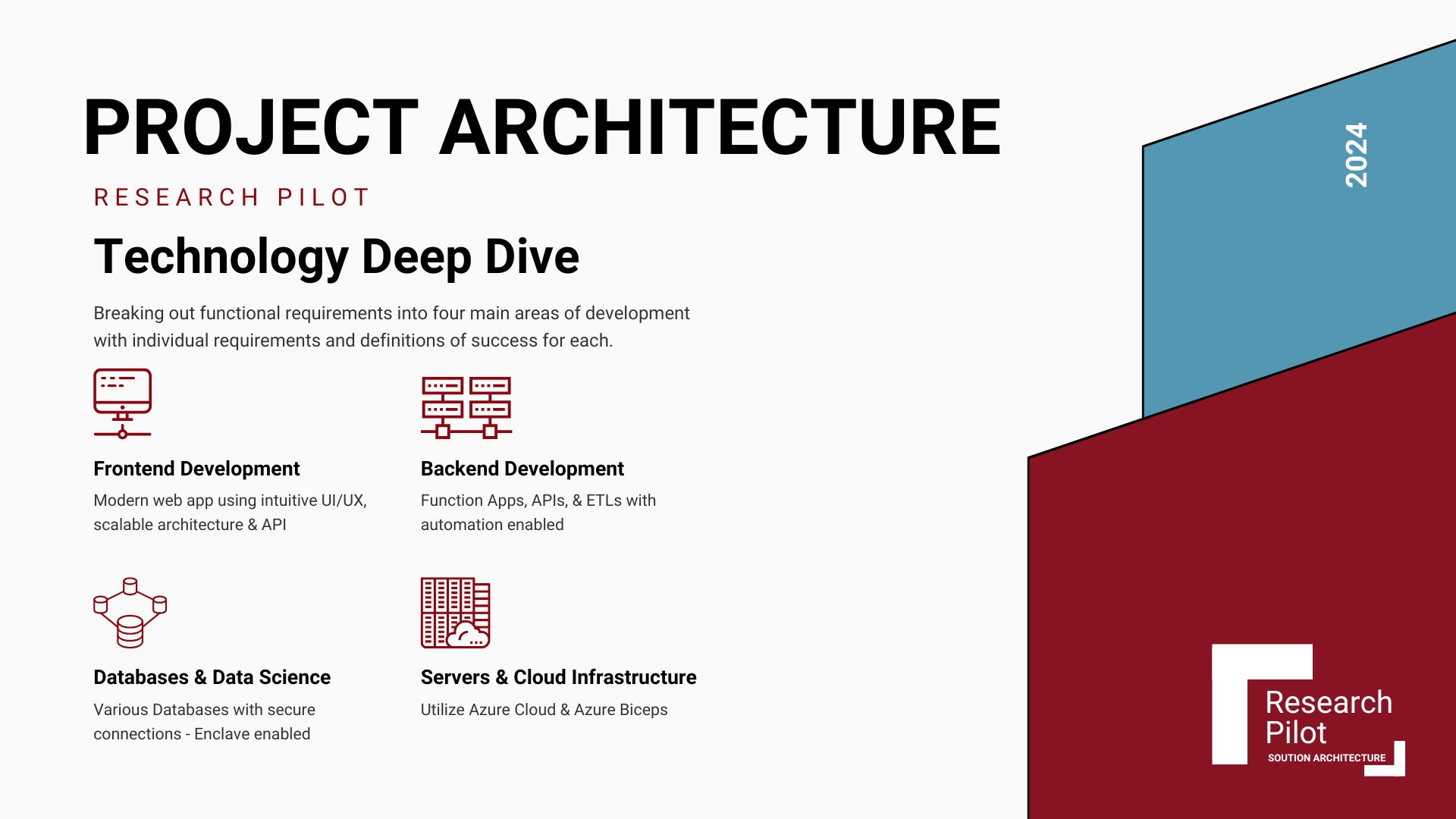
Architecture Documents
Technical architecture documentation acts as a reference point for development teams, enabling them to understand system design, dependencies, and constraints. It's crucial for onboarding new team members and maintaining consistency across the project's lifecycle.


Design Documents
Design documentation is a collection of documents that cover all the most important aspects of your product or service design. It keeps everyone on the same page, and if there's a new team member, it lets them know what's been done, why it's been done, how to implement certain elements, what's next, and more.


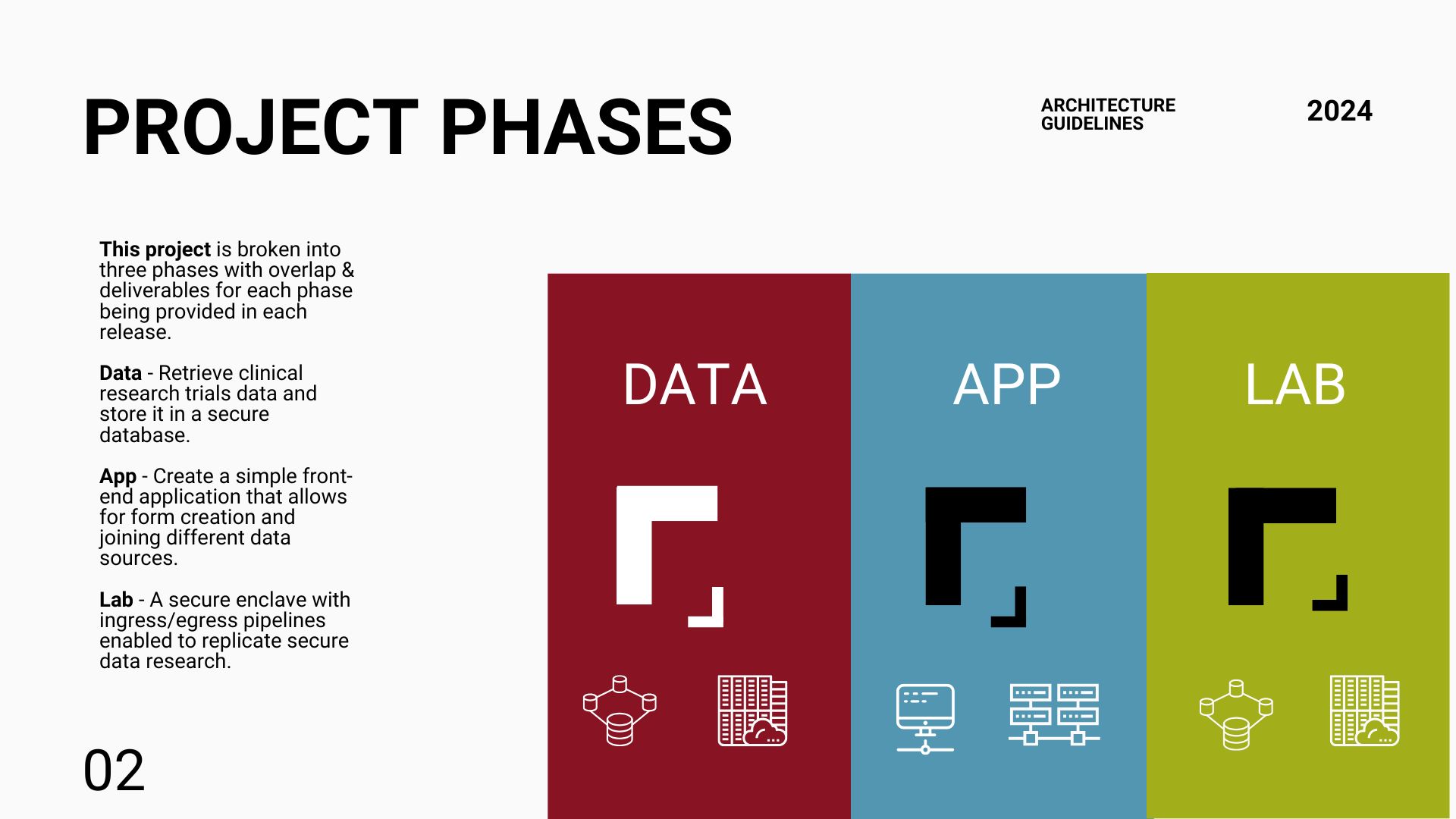
Phase One—MVP
We stood up this architecture and developed all the necessary modules to complete this proof-of-concept solution.